Product therapy and innovation

This is the turning point in hypertension care.
The Symplicity™ blood pressure procedure uses the Symplicity Spyral™ renal denervation system to help lower blood pressure along with lifestyle changes and medications. Results may vary.

Evolut™ FX+ TAVR system is designed for access.
Evolut™ FX+ TAV has larger windows to enable lifetime management solutions, such as coronary access,1 without compromising the known performance of the Evolut™ platform.2
Performance as compared to Evolut™ PRO+ and FX system in bench testing. Bench testing may not be indicative of clinical performance.

Together for venous patients
The Abre™ venous self-expanding stent system is designed for the unique challenges of venous disease. It offers easy deployment to let physicians focus on their patients and delivers demonstrated endurance to give patients freedom of movement.8,9

Less invasive, long-lasting
Designed for end-stage renal disease (ESRD) patients requiring hemodialysis, the Ellipsys™ system is a unique single-catheter, nonsurgical option for physicians to create an arteriovenous (AV) fistula, a traditionally invasive procedure that - until the advent of percutaneous AVF technology - had not changed in more than 50 years.

Reach new lengths with VenaSeal™ closure system.
Treat more diseased vein — maximize your impact. The VenaSeal closure system delivers immediate and lasting vein closure with its proprietary medical adhesive formula, with a demonstrated 94.6% closure rate at five years.3–7

Call in the reinforcements.
EndoSuture Aneurysm Repair (ESAR) with the Heli-FX™ EndoAnchor™ system is your defense for patients at risk for suboptimal outcomes.
- Reinforce the proximal seal12,13
- Protect against neck dilatation14
- Minimize type Ia endoleaks15
- Promote greater sac regression16
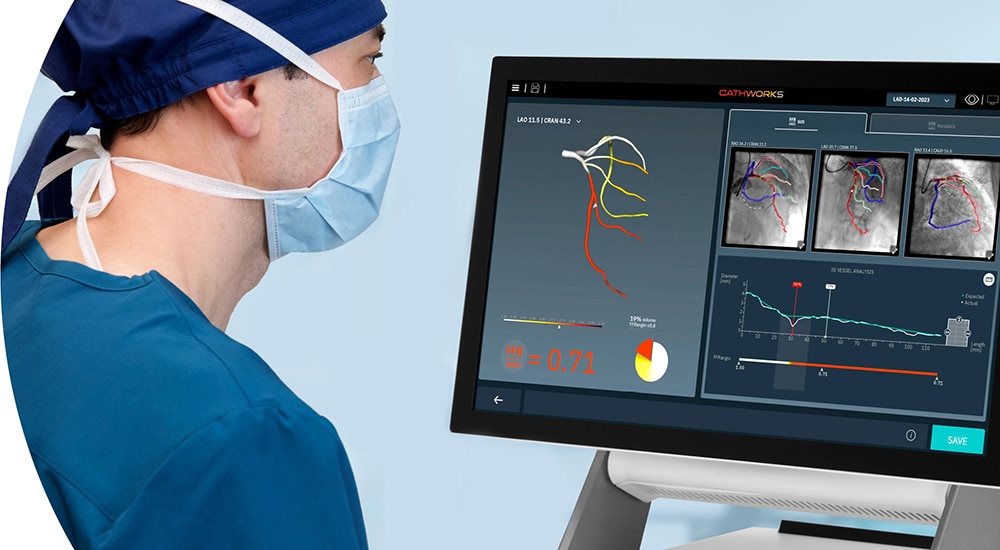
Partners for good measure
Our co-promotion agreement with CathWorks adds the CathWorks FFRangio®* System to our PCI portfolio. See how the technology's use of advanced artificial intelligence and computational science can help inform treatment decisions.
The best durability
Only CoreValve™/Evolut TAVR has shown a durability benefit over SAVR in multi-centered, randomized clinical trials out to 5 years10 and 10 years.11
Clinical evidence
IN.PACT™ AV Access clinical study 36-month results
The IN.PACT™ AV Access Study demonstrates the safety and effectiveness of the IN.PACT™ AV drug-coated balloon in arteriovenous fistulas.17 Learn more about the study and the 36-month results.
36-month results from the ABRE clinical study
The ABRE clinical study demonstrates the safety and effectiveness of the Abre venous stent.8 Learn more about the study and the 36-month results.
SMART Trial 1-year outcomes18
The SMART Trial data are clear: Evolut™ TAVR delivers superior valve performance† versus SAPIEN®* platform in small annulus patients‡ at one year.18 Learn more about this impactful trial.
ANCHOR wide neck data
ESAR reinforces and protects the proximal seal, leading to better outcomes in wide necks. View data on wide necks from literature compared to the three-year wide neck results from the ANCHOR Registry.
VenaSeal™ Spectrum Program: next-level evidence for venous care
The VenaSeal™ Spectrum Program is the largest post-market clinical study of the VenaSeal™ closure system compared to the current standards of care.
Education and training
Symplicity™ blood pressure procedure webinar series
Watch the series, which covers the challenges of achieving hypertension control, clinical data for renal denervation therapy, tips for incorporating the Symplicity™ procedure into an existing care pathway, and more.
New TAVR resources for HCP and patient education
Explore our new aortic stenosis and TAVR-related resources that will help you build your knowledge and support your patient education efforts in your heart valve clinic.
Aortic virtual education
As your partner in the evolving healthcare landscape, we've developed various webinar series for real-time and on-demand learning with peer-to-peer discussion.
Bifurcation education built for you
Visit Medtronic Academy to browse our latest resources, including step-by-step procedural videos of non-left main bifurcation using the provisional stenting technique.
Connect with us.
Follow us on social media.
- LinkedIn: Medtronic Cardiac & Vascular
Upcoming events
- TCT 2024
October 27–30, 2024
Additional resources
Resources
Cardiovascular technical support
Products
®* Third-party brands are trademarks of their respective owners.
† Valve performance as defined as freedom from bioprosthetic valve dysfunction (BVD) at 12 months. BVD is a composite including any of the following: hemodynamic structural valve dysfunction (mean gradient ≥ 20 mmHg), nonstructural valve dysfunction (severe prosthesis-patient mismatch or ≥ moderate aortic regurgitation), thrombosis, endocarditis, and aortic valve reintervention.
‡ In patients with small annuli (area ≤ 430 mm2) in all-comers trial, consisting of majority low surgical risk participants (52.1%).
TAVR risks may include, but are not limited to: death, stroke, damage to the arteries, bleeding, and the need for a permanent pacemaker.
- Based on Evolut™ FX+ Test Report: DO1106198 Rev. A. Medtronic computational data model on file compared to the Evolut™ platform. Bench top computational model may not be indicative of clinical performance.
- Based on Evolut™ FX+ Test Reports: D01073856, D01095344, D01084996. Performance as compared to Evolut™ PRO+ and FX system in bench testing. Bench testing may not be indicative of clinical performance. Medtronic data on file.
- Morrison N, Gibson K, McEnroe S, et al. Randomized trial comparing cyanoacrylate embolization and radiofrequency ablation for incompetent great saphenous veins (VeClose). J Vasc Surg. April 2015;61(4):985–994.
- Proebstle T, Alm J, Dimitri S, et al. Three-year follow-up results of the prospective European Multicenter Cohort Study on Cyanoacrylate Embolization for treatment of refluxing great saphenous veins. J Vasc Surg Venous Lymphat Disord. March 2021;9(2):329–334.
- Gibson K, Ferris B. Cyanoacrylate closure of incompetent great, small and accessory saphenous veins without the use of post-procedure compression: Initial outcomes of a post-market evaluation of the VenaSeal System (the WAVES Study). Vascular. April 2017;25(2):149–156.
- Almeida JI, Javier JJ, Mackay EG, Bautista C, Cher DJ, Proebstle TM. Thirty-sixth-month follow-up of first-in-human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. J Vasc Surg Venous Lymphat Disord. September 2017;5(5):658–666.
- Morrison N, Gibson K, Vasquez M, Weiss R, Jones A. Five-year extension study of patients from a randomized clinical trial (VeClose) comparing cyanoacrylate closure versus radiofrequency ablation for the treatment of incompetent great saphenous veins. J Vasc Surg Venous Lymphat Disord. November 2020;8(6):978–989.
- Abre™ stent instructions for use.
- Test data on file at Medtronic. Bench test results may not be indicative of clinical performance.
- O'Hair D, Yakubov SJ, Grubb KJ, et al. Structural valve deterioration after self-expanding transcatheter or surgical aortic valve implantation in patients at intermediate or high risk. JAMA Cardiol. February 1, 2023;8(2):111–119.
- Jørgensen T. Ten-year follow-up after transcatheter or surgical aortic valve implantation in severe aortic valve stenosis. Presented at ESC Congress; August 2023.
- Melas N, Perdikides T, Saratzis A, Saratzis N, Kiskinis D, Deaton DH. Helical EndoStaples enhance endograft fixation in an experimental model using human cadaveric aortas. J Vasc Surg. June 2012;55(6):1726–1733.
- Schlösser FJV, de Vries JPPM, Chaudhuri A. Is it time to insert EndoAnchors into routine EVAR? Eur J Vasc Endovasc Surg. April 2017;53(4):458–459.
- Tassiopoulos AK, Monastiriotis S, Jordan WD, Muhs BE, Ouriel K, De Vries JP. Predictors of early aortic neck dilatation after endovascular aneurysm repair with EndoAnchors. J Vasc Surg. July 2017;66(1):45–52.
- ANCHOR 4-year primary arm. 2019 data cut. Medtronic data on file.
- Muhs BE, Jordan W, Ouriel K, Rajaee S, de Vries JP. Matched cohort comparison of endovascular abdominal aortic aneurysm repair with and without EndoAnchors. J Vasc Surg. June 2018;67(6):1699–1707.
- Holden A. The IN.PACT AV Access Study: Results through 36 Months. Presented at Charing Cross 2022.
- Herrmann HC, Mehran R, Blackman DJ, et al. Self-Expanding or Balloon-Expandable TAVR in Patients with a Small Aortic Annulus. N Engl J Med. 2024. Online ahead of print. doi:10.1056/NEJMoa2312573.
