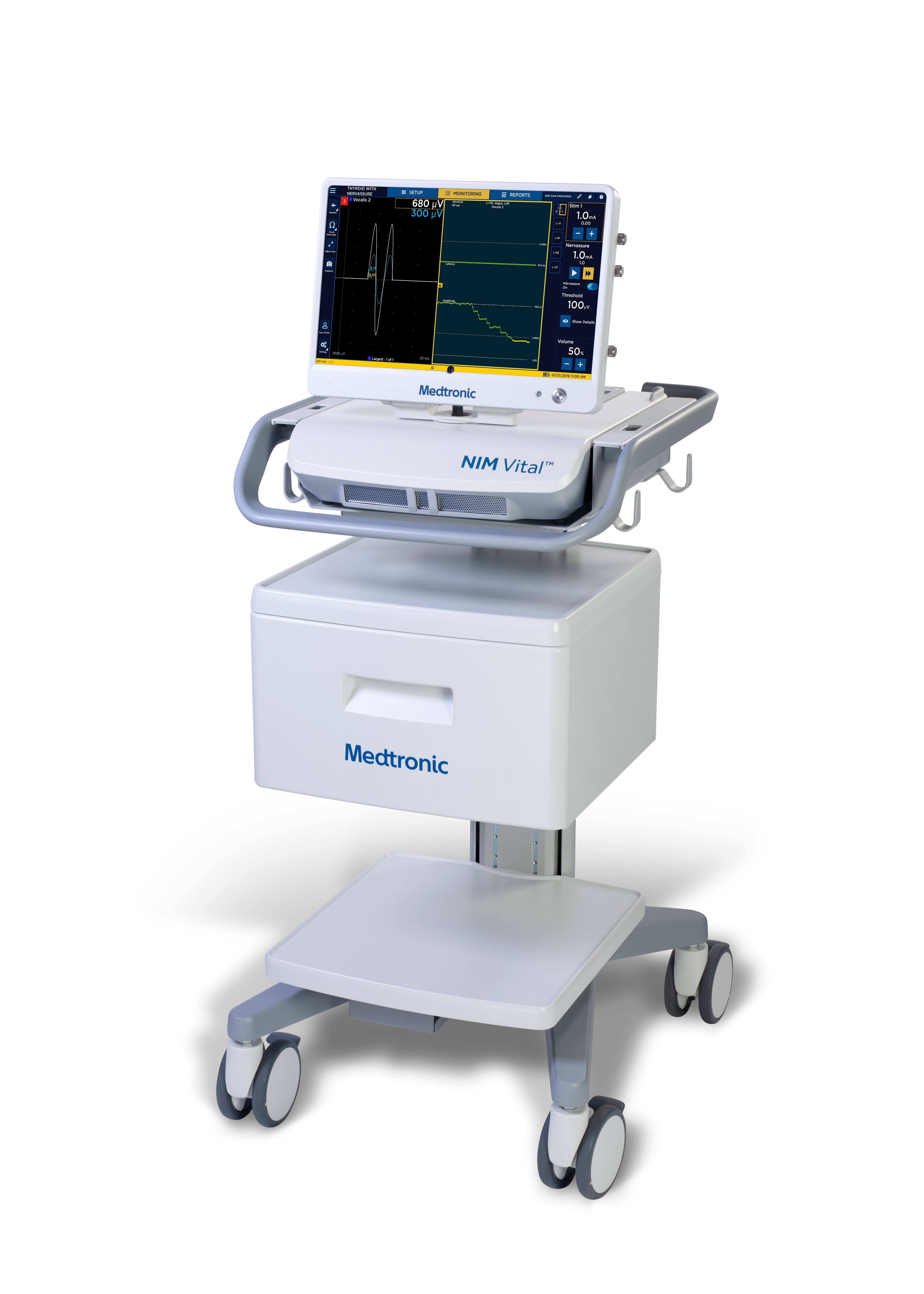
NIM Vital™ is the only system with NerveTrend™ and Nervassure™ EMG technology that inspires how you advance your surgical strategy1,2 and confidence3-4 to protect your patients' nerves and senses.5-6
WHY THE NIM VITAL SYSTEM
Protection is non-negotiable
Advance your nerve monitoring skills with the latest generation of nerve monitoring technology.

NerveTrend™ technology
A stimulator probe is used for manual trending during Intermittent monitoring.
More information (see more)
Less information (see less)
Nervassure™ technology
APS™ electrode is used for automatic stimulation during continuous monitoring.
More information (see more)
Less information (see less)
| Console | NIM Vital™ system | NIM™ 3.0 system |
|---|---|---|
| Simplified and easy-to-use user interface (UI)8 | √8 | X |
| Wireless monopolar muting9 | √9 | X |
| Wireless patient interface10 | √10 | X |
| Console battery as backup power11 | √11 | X |
| Software | NIM Vital™ system | NIM™ 30 system |
| Adjust12 | √12 | √ |
| NIM NerveTrend™13 | √13 | X |
| NIM Nervassure™ CIONM alarms and color coding (thyroid)14 | √14 | X |
| NIM Nervassure™ CIONM alarms and color coding (parotid)14 | √14 | X |
| Enhanced artifact noise suppression15 | √15 | X |
| Best channel16 | √16 | X |
| Increased stimulation range (up to 50mA)17 | √17 | X |
| Intelligent troubleshooting18 | √18 | X |
| Monitoring during bipolar cautery19 | √19 | X |
| Combination with a drill system (Stim bur guard)20 | √20 | √ |
| Recollection and overlay of previously saved waveform | √21 | √ |
35
years of nerve monitoring excellence worldwide
NIM VITAL™ REPAIR SERVICE PLAN
Peace of mind: Protect your capital investment
Medtronic recommends annual planned maintenance to promote system accuracy related to stimulation and monitoring. In December 2011, the U.S. Centers for Medicare and Medicaid Services (CMS) stated that “Manufacturer-recommended maintenance frequency is required for all equipment critical to patient health and safety”.7

Calò PG, Medas F, Conzo G, et al. Intraoperative neuromonitoring in thyroid surgery: Is the two-staged thyroidectomy justified? Int J Surg. 2017;41 Suppl 1:S13-S20. doi:10.1016/j.ijsu.2017.02.001
Fundakowski CE, Hales NW, Agrawal N, et al. Surgical management of the recurrent laryngeal nerve in thyroidectomy: American Head and Neck Society Consensus Statement. Head Neck. 2018;40(4):663-675. doi:10.1002/hed.24928.
Pardal-Refoyo JL, Parente-Arias P, Arroyo-Domingo MM, et al. Recommendations on the use of neuromonitoring in thyroid and parathyroid surgery. Recomendaciones sobre el uso de la neuromonitorización en cirugía de tiroides y paratiroides. Acta Otorrinolaringol Esp (Engl Ed). 2018;69(4):231-242. doi:10.1016/j.otorri.2017.06.005Statement. Head Neck. 2018;40(4):663-675. doi:10.1002/hed.24928.
Maneeprasopchoke P, Chongkolwatana C, Pongsapich W, Iwata AJ, Kamani D, Randolph GW. Intraoperative nerve monitoring in thyroid surgery: Analysis of recurrent laryngeal nerve identification and operative time. Laryngoscope Investig Otolaryngol. 2021;6(2):354-361. Published 2021 Feb 26. doi:10.1002/lio2.543.
Iwata AJ, Liddy W, Barczyński M, et al. Superior Laryngeal Nerve Signal Attenuation Influences Voice Outcomes in Thyroid Surgery. Laryngoscope. 2021;131(6):1436-1442. doi:10.1002/lary.29413
Thong, G, Brophy, C, Sheahan, P. Use of intraoperative neural monitoring for prognostication of recovery of vocal mobility and reduction of permanent vocal paralysis after thyroidectomy. Head & Neck. 2021; 43: 7–14. https://doi.org/10.1002/hed.26440.
U.S. Centers for Medicare and Medicaid Services (as accessed on January 20, 2020) https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/SCLetter12_07.pdf
Medtronic formative usability report, 10937733DOC, page 5, February, 2019.
NIM Vital™ final software system integration verification report 10963359DOC, attachment B, pages 69.
Iwata AJ, Liddy W, Barczyński M, et al. Superior Laryngeal Nerve Signal Attenuation Influences Voice Outcomes in Thyroid Surgery. Laryngoscope. 2021;131(6):1436-1442. doi:10.1002/lary.29413
NIM Vital™ IFU Pg. 52.
Design verification: NIM 4.0 software system verification Protocol #10918586DOC; NIM™ Vital™ claims matrix.
Design Verification: NIM 4.0 software system verification Protocol #10918586DOC.
NIM 4.0 software system verification Protocol #10918586DOC.
NIM Vital Artifact Rejection Verification, Report 10937825DOC, page 2, 2019.
NIM 4.0 Software System Verification Report, 10939013DOC, attachment B, page 9.
10960629DOC NIM4.0 Patient Interface 4-channel complete Design Verification report, Pg 51, 2020.
NIM Vital System Design Test Report 10960627DOC, Pages 41, 2020.
10960627DOC NIM4.0 System Verification report, Pg. 59, 2020. 24. NIM Vital IFU Pg. 58; Software validation.
10960627DOC NIM4.0 System Verification report, Pg. 59, 2020. 24. NIM Vital IFU Pg. 58; Software validation.
Design Verification: NIM 4.0 software system verification Protocol #10918586DOC.
