| Item number | Description | Shaft length (cm) | Units per box |
|---|---|---|---|
| LXMJ23S | LigaSure™ XP Maryland jaw sealer-divider with nano-coating, one-step sealing | 23 | 6 |
| LXMJ37S | LigaSure™ XP Maryland jaw sealer-divider with nano-coating, one-step sealing | 37 | 6 |
| LXMJ44S | LigaSure™ XP Maryland jaw sealer-divider with nano-coating, one-step sealing | 44 | 6 |
| LXMJ23L | LigaSure™ XP Maryland jaw sealer-divider with nano-coating, latching handle | 23 | 6 |
| LXMJ37L | LigaSure™ XP Maryland jaw sealer-divider with nano-coating, latching handle | 37 | 6 |
| LXMJ44L | LigaSure™ XP Maryland jaw sealer-divider with nano-coating, latching handle | 44 | 6 |
Laparoscopic instruments
LigaSure™ XP Maryland jaw sealer-divider with nano-coating
Laparoscopic instruments
LigaSure™ XP Maryland jaw sealer-divider with nano-coating
The LigaSure™ XP Maryland jaw device offers next-level vessel sealing technology − including latching handles and one-step sealing.
Description
Performance advantages that give you the edge.
The LigaSure™ XP Maryland jaw sealer-divider is advancing surgical energy to meet the evolving needs of surgeons and their patients. With the LigaSure™ XP Maryland jaw device, you get all the advantages of the LigaSure™ technology you’ve come to love, plus additional next-level benefits.
Features
Take vessel sealing to the next level.
Offering one-step or latching handle designs, LigaSure™ XP Maryland jaw devices provide surgeons new levels of performance,†,‡,§,1 precision,†,‡,1 and procedural efficiency‡,◊,1 in vessel sealing.
Performance
Increased jaw length and optimal force to manage thick tissue§,¶,2,3
- Longer seal plate with increased cut length#,∆,2
- Larger jaw opening (aperture)∞,4
- Higher jaw force for increased tissue compression when sealing and manipulating tissue5
- Nano-coated jaws reduce tissue sticking¶,6

Precision
Designed for more precise dissection††,#,2
- 360° continuous jaw rotation
- Energized tissue dissection to tip of device††,#,2
- Blade travels close to device tip††,#,2
- Curved, tapered jaw profile for fine dissection††,‡‡,2
- Jaw design makes it easier to access tissue planes‡‡,§§,2

Procedural efficiency
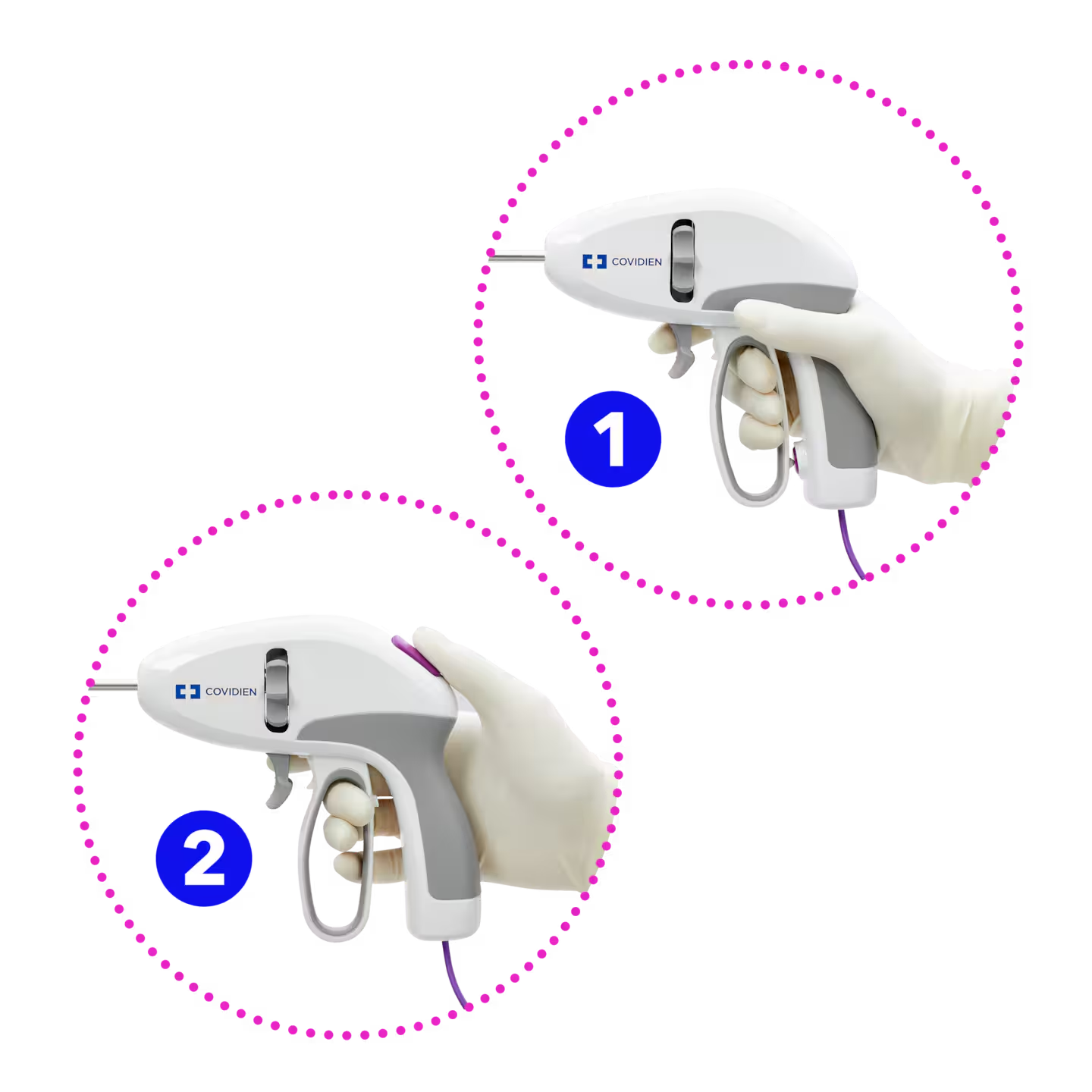
Ergonomic handle5 in both latching and one-step sealing options
- One-step sealing handle option provides tactile response before contacting activation button§§,4
- Latching handle option provides distinct and separate actions to grasp, seal, and cut with less hand fatigue◊ ◊,2
Specifications
| Physical characteristics | |
|---|---|
| General | Single use |
| Handle designs | One-step sealing or latching handle |
| Shaft lengths (cm) | 23, 37, and 44 |
| Shaft rotation | Continuous 360° jaw rotation |
| Jaw length (mm) | 23.4 |
| Seal plate length (mm) | 22.6 |
| Cut length (mm) | 21.8 |
| Device weight (g) | 262 |
| Shaft diameter (mm) | 5 |
| Foot-switching | yes |
Ordering information
Related links
Related products
Similar products
-
 LigaSure™ Maryland jaw thoracic sealer-divider with nano-coating
LigaSure™ Maryland jaw thoracic sealer-divider with nano-coatingThe LigaSure™ Maryland jaw thoracic sealer-divider is a multifunctional nano-coated instrument designed for sealing pulmonary veins and arteries.
-
 LigaSure™ retractable L-hook laparoscopic sealer-divider
LigaSure™ retractable L-hook laparoscopic sealer-dividerThe LigaSure™ retractable L-hook laparoscopic sealer-divider combines the functionality of five laparoscopic devices into one versatile instrument.
TM* Third-party brands are trademarks of their respective owners.
† Compared to their current preferred device; 21 of 23 surgeons agreed during clinical procedures.
‡ The LigaSure™ XP Maryland jaw device is indicated for use in general surgery and such surgical specialties as colorectal, bariatric, urologic, vascular, thoracic, and gynecologic.
§ Thick tissue is defined as nondissected vascular tissue or fatty tissue.
◊ Compared to their current preferred device; 20 of 23 surgeons agreed during clinical procedures.
¶ Bench tissue may not be indicative of clinical performance.
# Compared to legacy LigaSure™ devices
∆ 28 out of 29 surgeons agree.
∞ LigaSure™ XP Maryland jaw length and aperture (23.368 mm and 14.437 mm) versus LigaSure™* Maryland jaw length and aperture (20.523 mm and 12.344 mm) as measured from device tip to tissue stop.
†† 24 out of 29 surgeons agree.
‡‡ 29 out of 29 surgeons agree.
§§ 15 out of 15 surgeons agree.
◊◊ 14 out of 14 surgeons agree.
- Based on internal report RE00457416 rev A, Product introduction report: LigaSure™ XP Maryland jaw sealer-divider. May 2023.
- Based on internal report RE00442444 rev A, Comparison of the renal artery bench bundle burst pressure performance with the LigaSure™ XP Maryland jaw sealer-divider, Voyant™* Maryland Fusion, Enseal™* X1 curved jaw, and LigaSure™ LF19XX devices. January 23, 2023.
- Based on internal report RE00372649 rev A, Archer surgeon summative evaluation report. March 22, 2022.
- Based on internal test report RE00455509 rev B, Competitive device analysis — LigaSure™ XP Maryland jaw sealer-divider.
- Based on internal report RE00279542 rev A, LigaSure™ XP Maryland ergonomic improvements.
- Based on internal report RE00380835 rev B, Sticking evaluation with the LigaSure™ XP Maryland jaw sealer-divider, Aesculap Caiman™* 5 Maryland, Voyant™* Maryland Fusion, Ethicon Enseal™* X1 curved jaw, LigaSure™ LF18XX blunt tip, and LF19XX Maryland devices.




