| Item number | Description | Units per box |
|---|---|---|
| RESPARRAYAHA01 | RespArray™ patient monitor — AHA (American Heart Association) version | 1 |
| RESPARRAYKITAHA | RespArray™ patient monitor bundle | 1 |
Continuous patient monitoring
Continuous patient monitoring
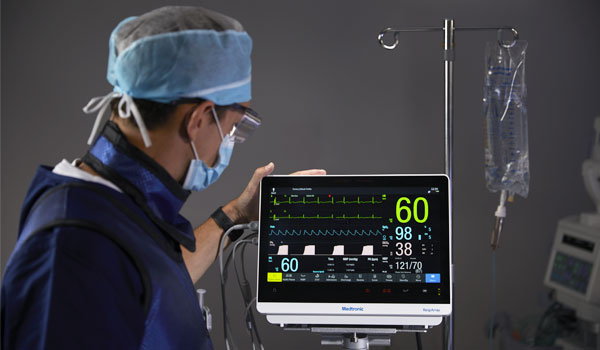
The RespArray™ patient monitor can help continuously monitor SpO₂, etCO₂, ECG, and temperature, measure NiBP, and connect wirelessly to EMR via HL7.
We have an unwavering commitment to keep our patients safe. But staffing shortages can make it challenging to check on your patients as often as you’d like.
What if you could monitor patients from anywhere in the hospital? Now you can, with the the RespArray™ patient monitor.
It's designed to continuously monitor patients in areas of care where spot checking might not be enough or where additional parameters are needed — for example, in medical-surgical units or for capnography use in procedural sedation. The RespArray™ patient monitor can help you detect respiratory compromise early and intervene sooner.
The RespArray™ patient monitor features simple connectivity and seamlessly integrates into workflow. You’ll have more time to focus on your patients.
96%
On average, medical-surgical registered nurses spend 96% of their shift away from the patient’s bedside.1
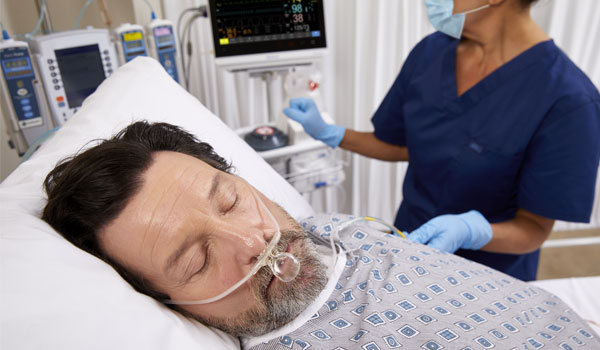
Experience world-class Nellcor™ pulse oximetry and Microstream™ capnography technologies, designed to detect respiratory compromise early — and help reduce alarm fatigue.
53%
The Smart Alarm for Respiratory Analysis™ (SARA) in Microstream™ technology reduces unnecessary nuisance alarms by 53%.2
Your dedicated white-glove service team is with you every step of the way. Customizable plans and service options are tailored to fit your needs.
$535K
Average amount that continuous monitoring of high-risk patients may help a median-sized hospital save annually†,3

| Item number | Description | Units per box |
|---|---|---|
| RESPARRAYAHA01 | RespArray™ patient monitor — AHA (American Heart Association) version | 1 |
| RESPARRAYKITAHA | RespArray™ patient monitor bundle | 1 |
Select a module below to watch an interactive video.

Module 1: Why the RespArray™ patient monitor?

Module 2: Overview of the RespArray™ patient monitor

Module 3: RespArray™ patient monitor interface

Module 4: RespArray™ patient monitor parameters

Module 5: RespArray™ patient monitor workflow overview

Module 6: Alarms and troubleshooting
The measurements provided through the RespArray™ patient monitor, including Nellcor™, Microstream™, and other vital signs, should not be used as the sole basis for diagnosis or therapy and are intended only as adjuncts to patient assessment.
† This assumes a 20% respiratory depression reduction and an annual general care floor volume of 2,447 patients receiving opioids per median-sized hospital. 90% of surgical patients and 45% of medical patients on U.S. general care floors receive opioids. Continuous pulse oximetry and capnography device pricing assumptions used list pricing for the following: a Capnostream™ 35 portable respiratory monitor prorated over seven years; a Microstream™ capnography filter line, and a disposable Nellcor™ pulse oximetry sensor, resulting in $52.73 in device costs per continuously monitored patient stay on a medical surgical floor. For intermittent pulse oximetry monitoring, device pricing consisted of a multiparameter monitor prorated over seven years and a reusable pulse oximetry sensor, resulting in $0.68 in device costs per patient stay. Additional information on pricing and assumptions are available in the study publication.