
The PROPEL™ mometasone furoate family of implants
Connect with us
The PROPEL™ family of implants release mometasone furoate and have been shown to reduce inflammation and scarring leading to a reduction in the need for postoperative surgical and/or oral steroid interventions.1-3
The PROPEL™ family on implants offers 3 steroid-eluting implants under one brand, allowing ENTs to select the best option that conforms to the anatomical needs of their patients.
PROPEL™ mometasone furoate implant
For ethmoid sinus1
PROPEL™ Mini mometasone furoate implant

For ethmoid sinus and frontal sinus opening2
PROPEL™ Contour mometasone furoate implant

For sinus ostia:
frontal and maxillary3
The AAO and ARS have updated their position statements on drug-eluting sinus implants.
The American Academy of Otolaryngology-Head and Neck Surgery and the American Rhinologic Society consider drug-eluting implants in the paranasal sinuses as a proven and effective therapeutic option for mucosal inflamation.
Explore how PROPEL implants can improve postsurgical outcomes in your patients.
PROPEL™
in-office implant options
- In-office sinus procedures:
Implants following in-office ESS such as balloon sinus dilation - Postoperative utilization:
Implants during the postoperative healing period after sinus surgery
Complete your in-office action plan. Download plan
Ethmoid sinus surgery
(primary endpoint)‡,4
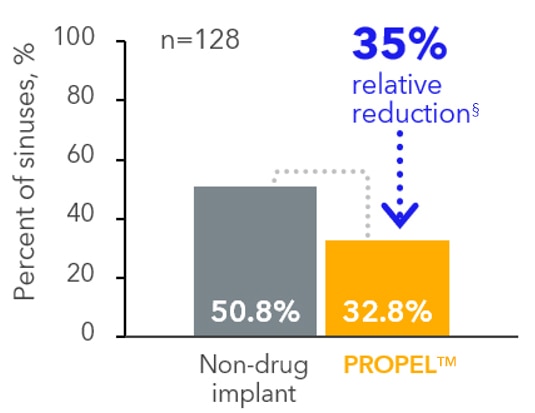
PROPEL™ implant delivered clinically significant reductions, regardless of nasal polyps, compared to a
non-drug implant
Frontal sinus surgery
(primary endpoint)‡,5


CRSwNP patients‡
(32.5% versus 50.6%; n=91)
Frontal sinus surgery6,7
(primary endpoint)
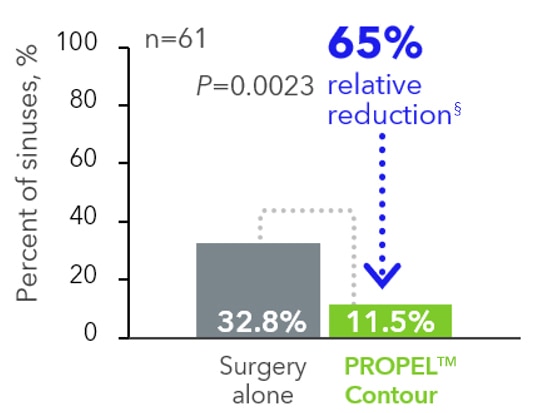

CRSsNP patients‡
(33.3% versus 51.1%; n=52)
§ Calculated as (T-C)/C as part of ad hoc analysis.
Your action plan for ethmoid sinus Download action plan
Your action plan for frontal sinus Download info
Patients with and without polyps
PROPEL™ mometasone furoate sinus implants significantly benefited patients with and without polyps.||4

relative reduction¶ in the need for postoperative intervention in patients without polyps (33.3% vs 51.1%; P=0.0455; n=52)

relative reduction¶ in the need for postoperative intervention in patients with polyps (32.5% vs 50.6%; n=91)
Patients with balloon, traditional, or hybrid surgery
PROPEL™ Contour implant patient breakdown#7: In the progress study, PROPEL™ Contour implants demonstrated a 63% relative reduction in occlusion/restenosis.
#Calculated as (T-C)/C as part of adhoc analysis.
19% Balloon dilation only
53% Traditional surgery
29% Combination of the two
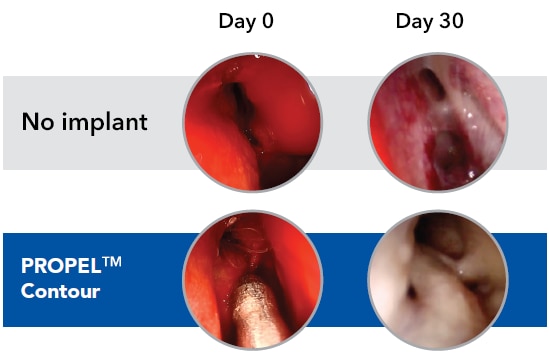
In a small subset of patients that received balloon dilation alone, the PROPEL™ Contour group had a 7.3 mm frontal sinus ostia vs 5.1 mm in the control group without PROPEL Contour at Day 30. No inferential statistical analyses were performed.
Example of a non-polyp patient**

Example of a balloon-only patient**
The PROPEL sinus implants are intended to maintain patency and locally deliver steroid to the sinus mucosa in patients ≥18 years of age following sinus surgery: PROPEL for the ethmoid sinus, PROPEL Mini for the ethmoid sinus/frontal sinus opening, and PROPEL Contour for the frontal/maxillary sinus ostia. Contraindications include patients with confirmed hypersensitivity or intolerance to mometasone furoate (MF) or hypersensitivity to bioabsorbable polymers. Safety and effectiveness of the implant in pregnant or nursing females have not been studied. Risks may include, but are not limited to, pain/ pressure, displacement of the implant, possible side effects of intranasal MF, sinusitis, epistaxis, and infection.
See full prescribing information (IFU).
Rx only.
Instructions For Use
PROPEL™: US | EU | Patient Implant Information Leaflet (EU)
PROPEL™ Mini: US | EU | Patient Implant Information Leaflet (EU)
PROPEL™ Mini with SDS: US
PROPEL™ Contour: US | EU
† Post-operative interventions is a composite of need for surgical interventions to separate adhesions and/or need to prescribe oral steroids for inflammation.
‡ Judged by a centralized, independent, blinded reviewer or panel.
§ Calculated as (T-C)/C as part of ad hoc analysis.
|| Patients with unilateral or bilateral polyps at baseline were grouped as polyp patients.
¶ PROPEL study design: Meta-analysis of two prospective, randomized, double-blinded multicenter studies (Pilot and ADVANCE II) that enrolled 143 patients.
# Calculated as (T-C)/C as part of adhoc analysis.
** PROPEL Contour study design: Randomized, controlled, double-blinded clinical trial (PROGRESS study) that enrolled 80 patients.
PROPEL [Instructions for Use]. Menlo Park, CA: Intersect ENT; 2020.
PROPEL Mini [Instructions for Use]. Menlo Park, CA: Intersect ENT; 2020.
PROPEL Contour [Instructions for Use]. Menlo Park, CA: Intersect ENT; 2020.
Han JK, et al. Int Forum Allergy Rhinol. 2012;2(4):271-279. Study design: Meta-analysis of two prospective, randomized, intra-patient controlled, double-blinded multicenter studies (Pilot and ADVANCE II) that enrolled 143 patients.
Smith TL, et al. Laryngoscope. 2016;126(12):2659-2664.
PROGRESS (Nova Cohort) CSR R500-0514, Rev 4.0, December 28, 2016.
Luong A, et al. JAMA Otolaryngol Head Neck Surg. 2018;144(1):28-35. Study design: A prospective, randomized, controlled, blinded clinical trial with two consecutive cohorts, evaluating outcomes of frontal sinus surgery (using balloons and/or traditional instruments) with PROPEL Mini (N=80) and PROPEL Contour (N=80) compared to surgery alone, both with standard postoperative care.