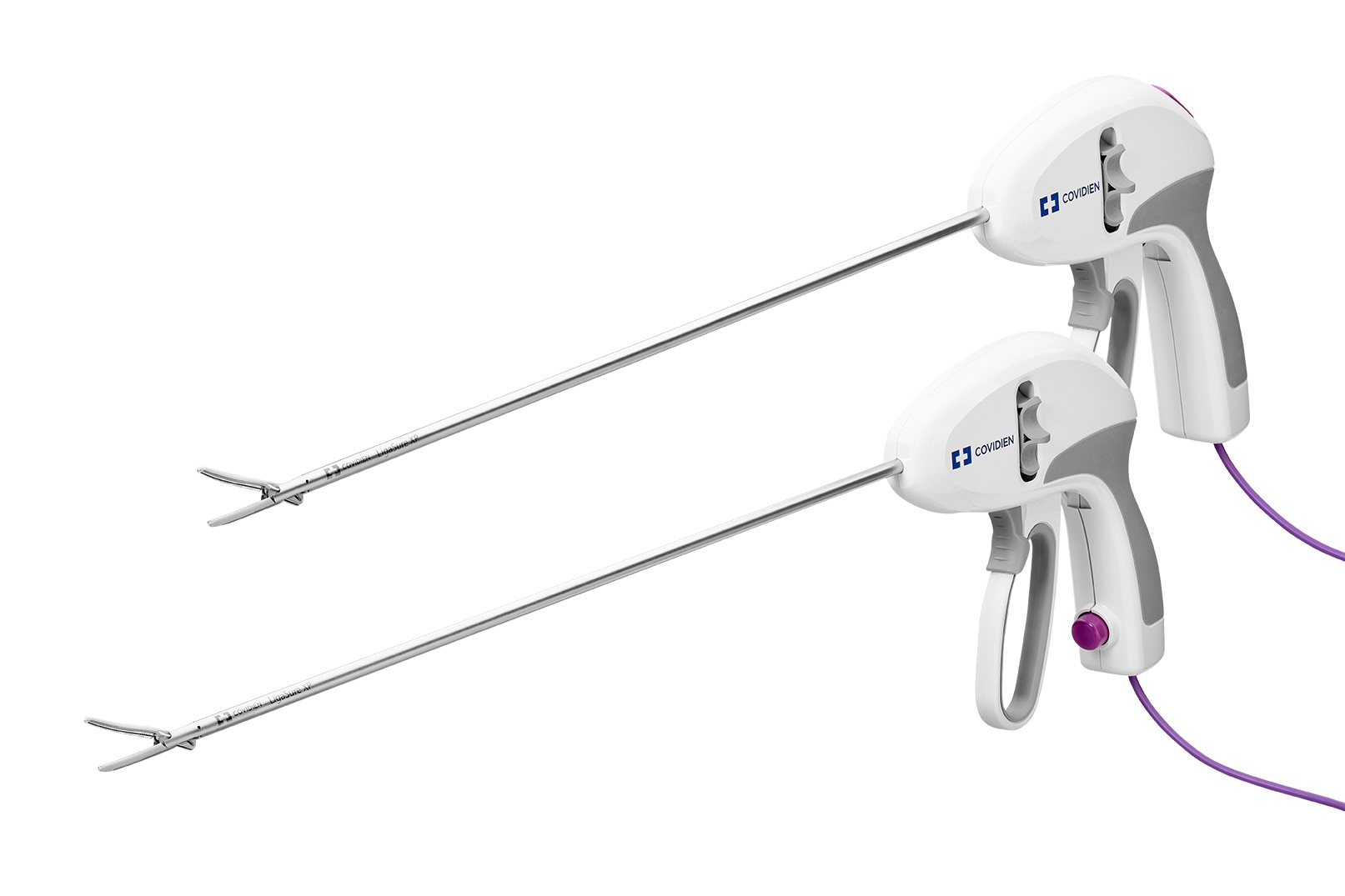
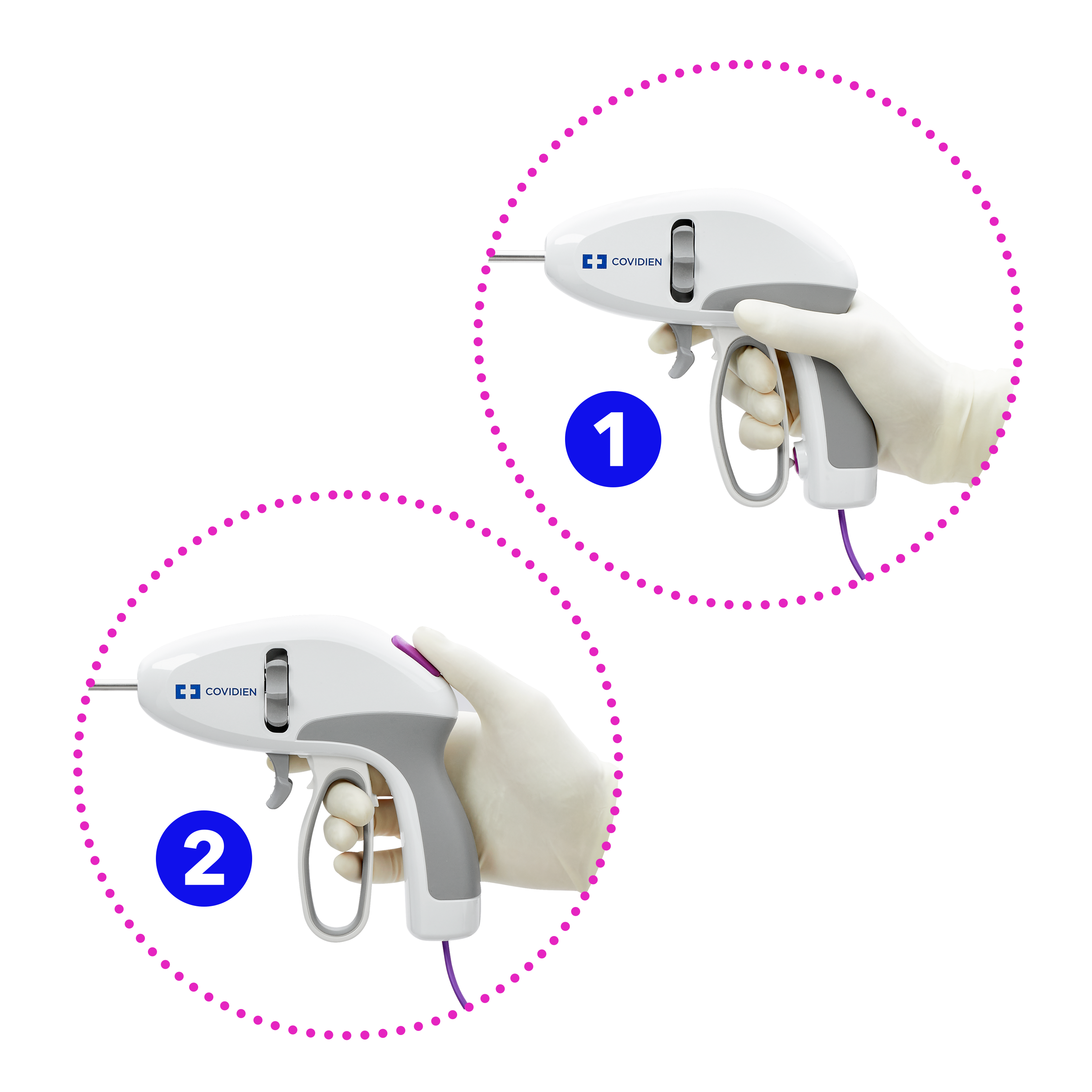
Select your country/language
- New Zealand
- Argentina
- Australia
- Belgique (Belgium) - Français
- België (Belgium) – Nederlands
- Brasil (Brazil)
- Canada – English
- Chile
- Colombia
- Danmark (Denmark)
- Deutschland (Germany)
- España (Spain)
- France – Français
- Italia (Italy)
- Japan - 日本
- Magyarország (Hungary)
- México
- Nederland (Netherlands)
- Norge (Norway)
- Panamá
- Perú
- Poland
- Portugal
- Puerto Rico
- Schweiz (Switzerland) - Deutsch
- South Africa
- Suisse (Switzerland) – Français
- Suomi (Finland)
- Sverige (Sweden)
- Svizzera (Switzerland) – Italiano
- Türkiye (Turkey)
- United Kingdom
- Österreich (Austria)
- Česká republika (Czech Republic)
- Ελλάδα (Greece)