References
†Compared to ETHIBOND EXCEL®* size 0.2-0, 2, and 5. May not be indicative of clinical performance.
‡Compared to ETHIBOND EXCEL®* size 2-0. May not be indicative of clinical performance.
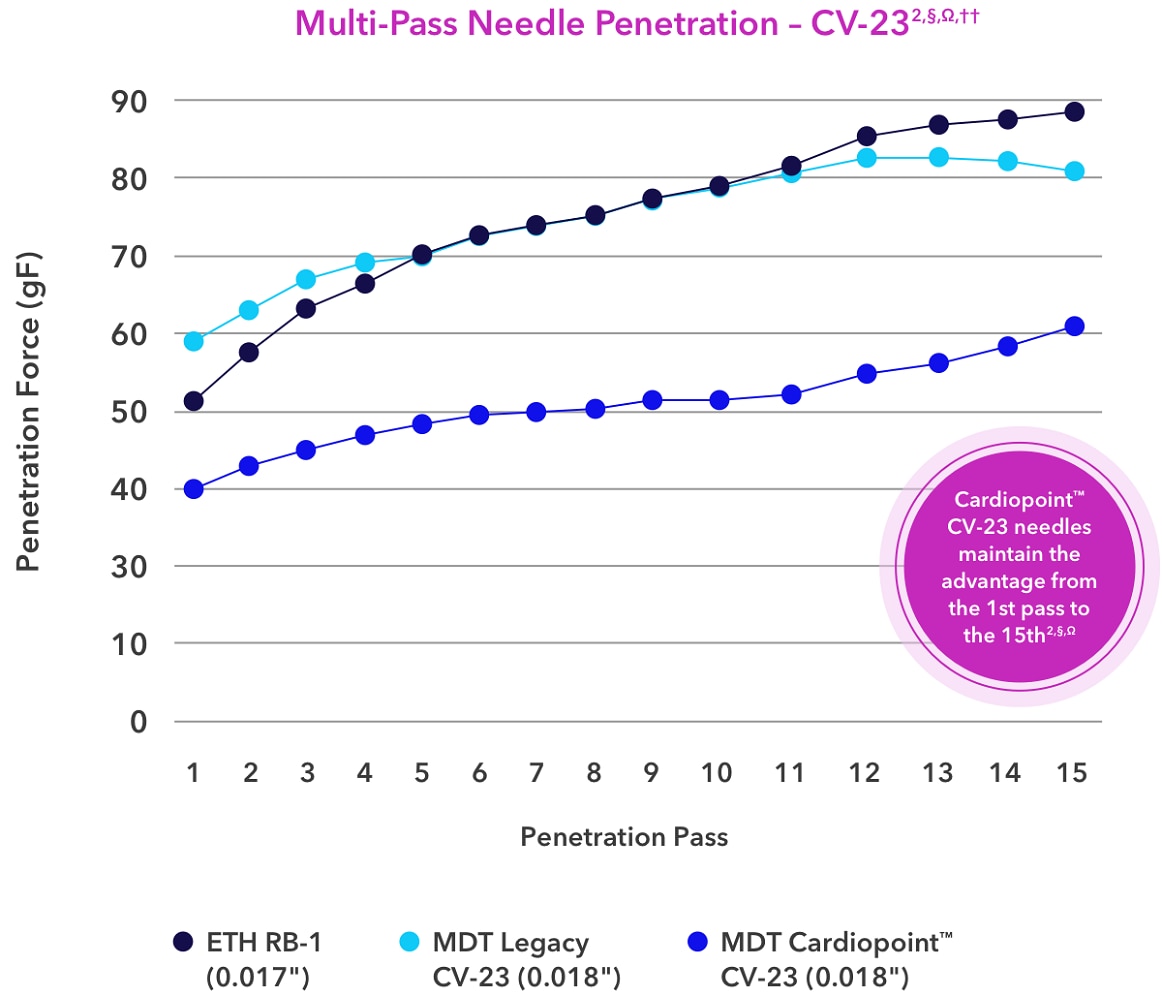
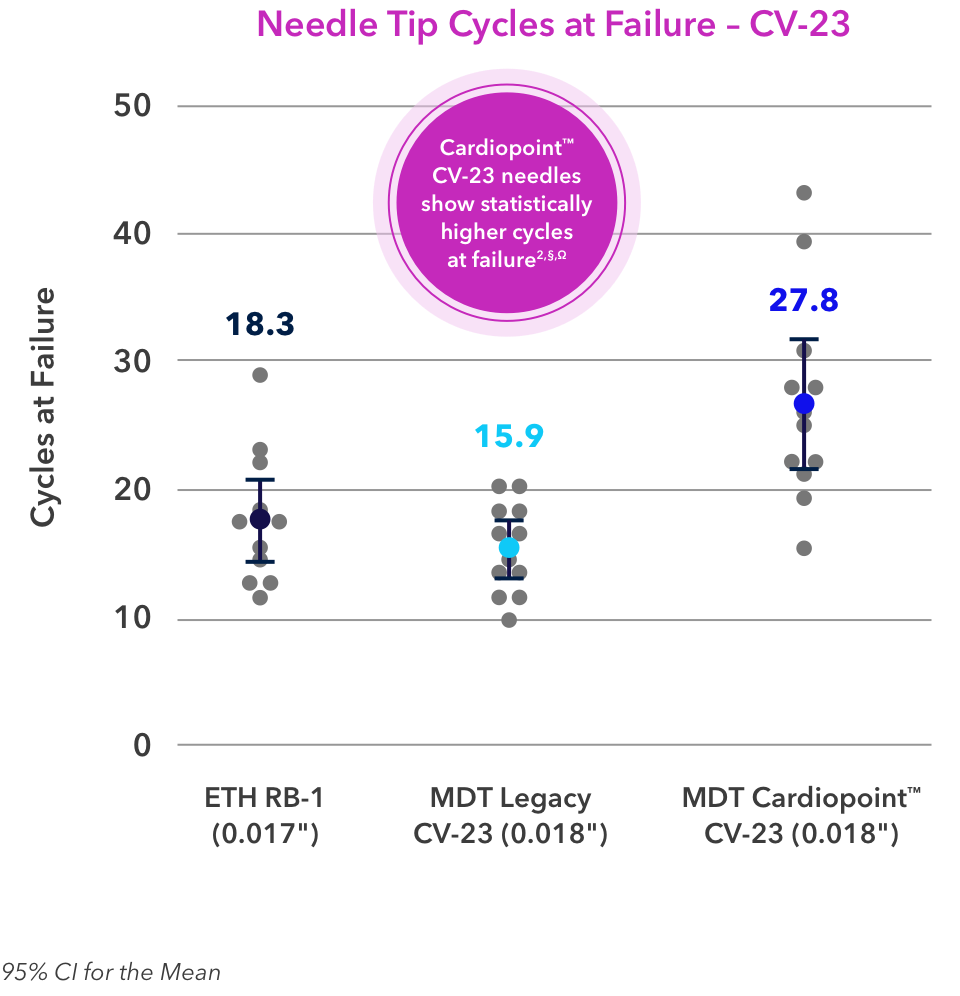
§Compared to Ethicon BB, RB1, and SH2 needles.
Ω Based on surgeon VOC lab, may not be indicative of clinical performance.
††Compared to legacy Medtronic needles with Surgalloy, may not be indicative of clinical performance.
‡‡Compared to previous generation of Surgipro™ sutures made with Poly (Ethylene Glycol) Distearate; measured by straight pull strength.
§§Based on benchtop test data; may not be indicative of clinical performance
ΩΩBased on surgeon double blinded VOC porcine tissue lab; may not be indicative of clinical performance.
†††Over rectangular pledgets.
‡‡‡Bench test data. May not be indicative of clinical performance.
1. Based on internal test report #RE00100396, Ti-Cron™ suture 2-0 knot security benchmark testing. August 19, 2015. CL-007061.
2. Based on validation report #RE00172196, Sharpie II Cardiopoint™ Needle Benchmarking. November 19, 2018.
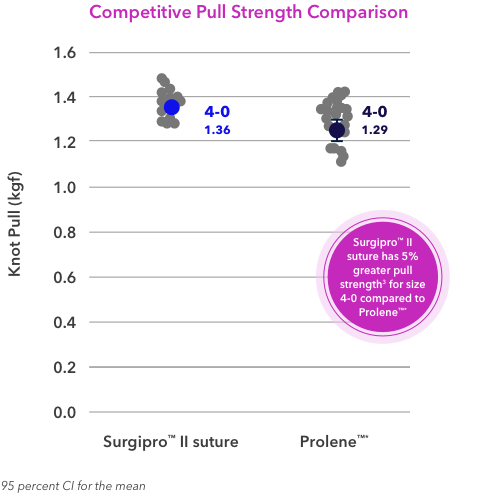
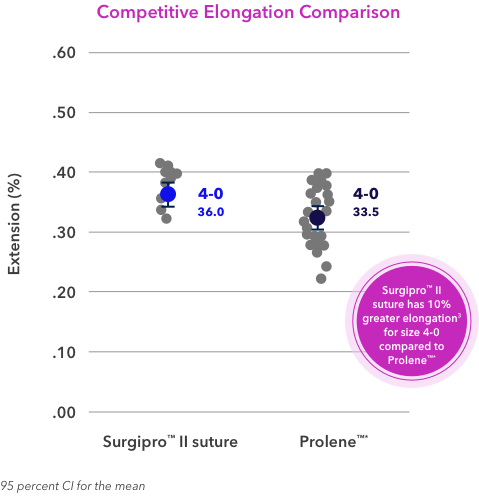
3. Based on validation report #RE00073496, Optimized Diameter Surgipro™ II 4-0 Evaluation. 01/24/2017. Based on validation report #RE00115198, Surgipro™ II 4-0 Top Performing DOE Sample Validation. 10/10/2017. Based on validation report #RE00167893,
Surgipro™ II 5-0 DOE Sample Validation. 09/04/2018. Based on validation report #RE00167894, Surgipro™ II 6-0 DOE Sample Validation. 09/04/2018. Based on validation report #RE00167895, Surgipro™ II 7-0 DOE Sample Validation. 09/25/2018.
4. Based on survey of 6 CV surgeons in two cities - NYC and Chicago. HMV Report. Pledget. Usability lab focus group, conducted on June 10, 2015. — 06-10-15 , 06-10-16, 06-10-17, 06-10-18, 06-10-20, 06-10-22, 06-10-25, 06-10-28.
5. Based on validation report #RE00171206 with 13 Surgeon Evaluation of Surgipro™ II CV Suture Strength. 09/18/2018.
6. Based on internal test report #RE00094876, Ti-Cron™ suture 2-0 knot security benchmark testing. August 19, 2015. CL-007062.
7. Based on validation report #re00073496, optimized diameter Surgipro™ II 4-0 evaluation. 24th January, 2017. Based on validation report #RE00115198, Surgipro™ II 4-0 top performing DOE sample validation. 10/10/2017.
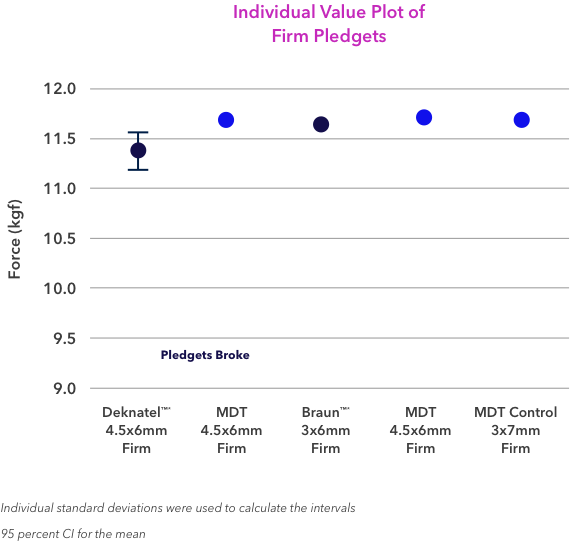
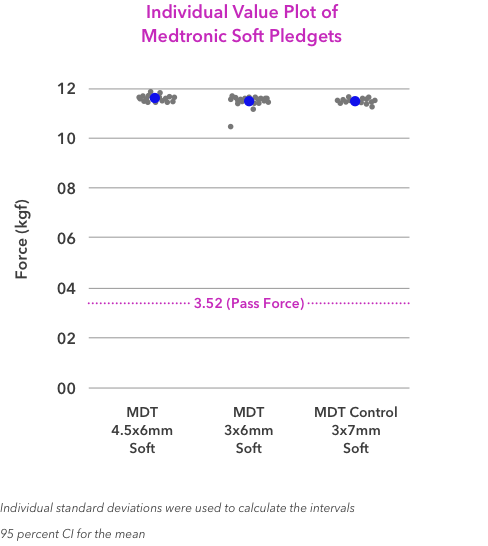
8. Medtronic R&D Engineering Report. Report Number: RE00017984. Determination of Pledget Removal Force. Aug. 7, 2015.