Overview
Given the current market situation and its consequences such as product recalls, supply chain disruptions and portfolio changes, we are pleased to introduce you to a potential alternative product in our respiratory portfolio to offer you.
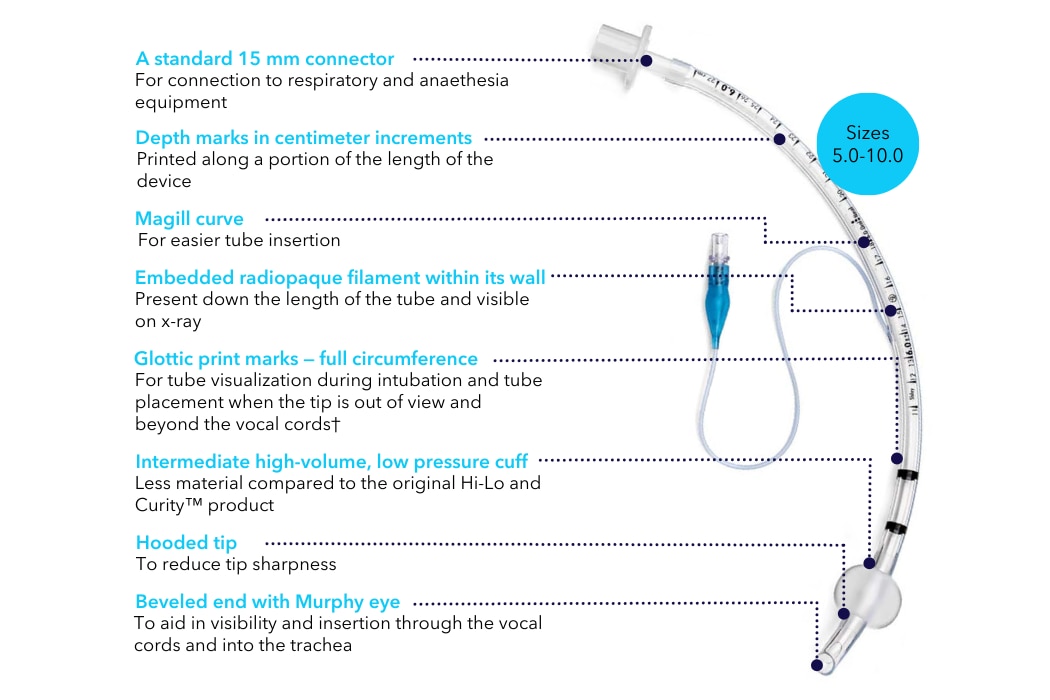
The Shiley™ oral/nasal endotracheal tube with intermediate cuff can be used for paediatric through adult patients.
- Size options 3.0–4.5 mm feature an intermediate low-profile cuff to meet the unique needs of pediatric patients
- Size options 5.0–10.0 feature an intermediate high-volume, low-pressure cuff
DEHP is very toxic and hazardous to organism health and its exposure has been shown to have many adverse effects.¹ Compared to the legacy version, the Shiley™ oral/nasal endotracheal tube with intermediate cuff is made with non-DEHP PVC material, which softens at body temperature and molds to the airway.